Which Best Describes the Properties of Representative Elements
These four sets of elements correspond to the properties of four actual groups on the Periodic Table. A Al - 3 b Br - 7.
2 5 The Periodic Table Chemistry
B located in the outermost occupied major energy level.

. In this article we will discuss periodic properties and their trends in. 206 Properties of Representative Elements 2348. D located in d orbitals.
204 Ionization Energy 1429. What best describes the properties of representative elements. Which of the following correctly describe atomic radii of representative elements.
Terms in this set 15 representative elements. Groups 3-12 1-2 electrons in the outer energy level less reactive than alsali-earth metals shiny good conductor of thermal energy and electrical current high density. They have the same number of valence electrons.
Which statement accurately describes a pattern in the size of atomic radii in the Periodic Table of the Elements. The Transition Metals are the elements in those Groups within the middle of the Table. Thus we can say that elements having similar electronic configurations have similar properties.
1 Atomic radii decrease as the effective nuclear charge decreases 2 Atomic radii generally increases as n increases 3 Atomic radii generally decrease down a group 4 atomic radii of main group elements decrease across a period. Usually displayed in the main body of a periodic table. They display a wide range of properties including metallic character and state of matter.
Alkaline earth metals d. Which element has the largest atomic radius. Answer choices Atomic radii decrease from left to right across a period and decrease from top to bottom in a group.
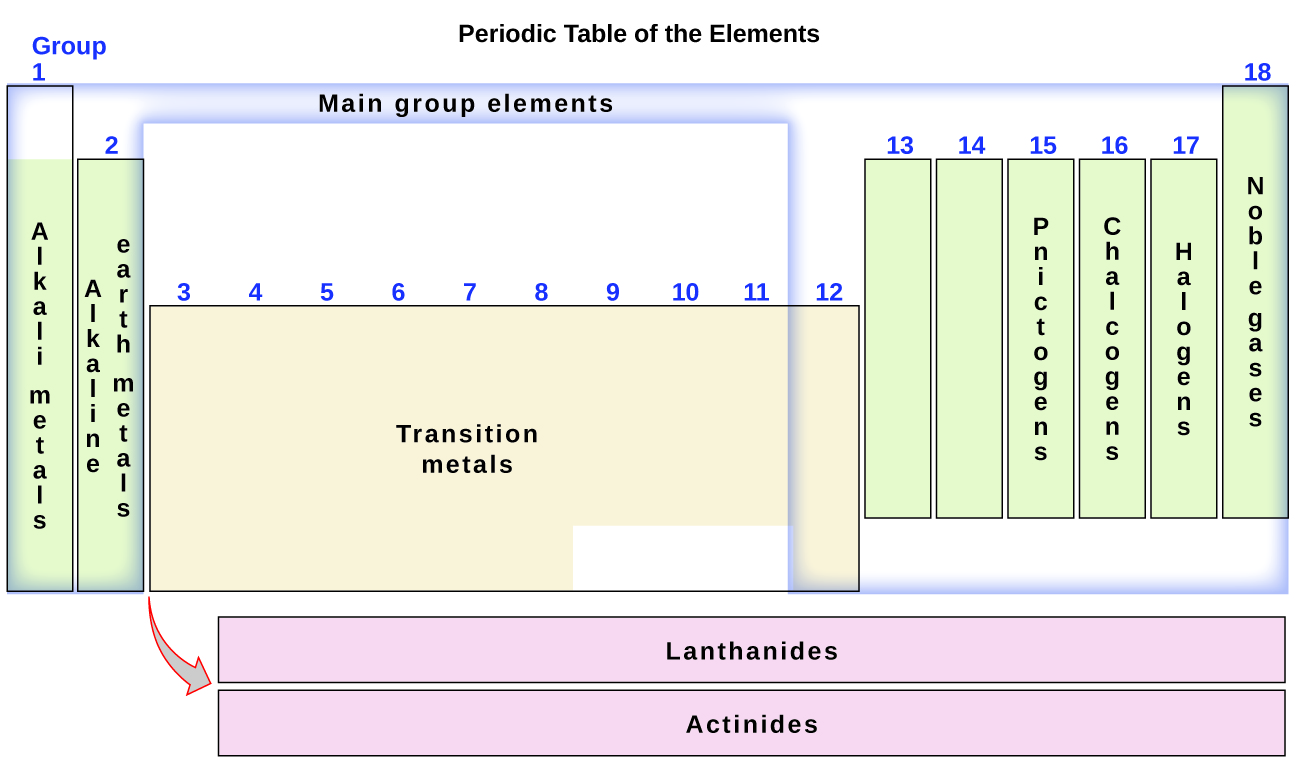
The chart below describes the properties of four sets of elements. The elements within the first two families Groups I and II on the far left and the last six families or groups on the right of the Periodic Table are known as representative elements. 204b Hydrogen-like atom of flourine 759.
Transition elements include d block and f block elements. We will describe some of the chemistry of the representative elements showing as we do so how their properties may be rationalized on the basis of concepts and principles. The alkali metals are soft lustrous metals that are highly conductive.
Some elements in these groups are metals some are nonmetals and some are metalloids. They have valence electrons in different energy levels. The valence electrons of representative elements are a in s orbitals only.
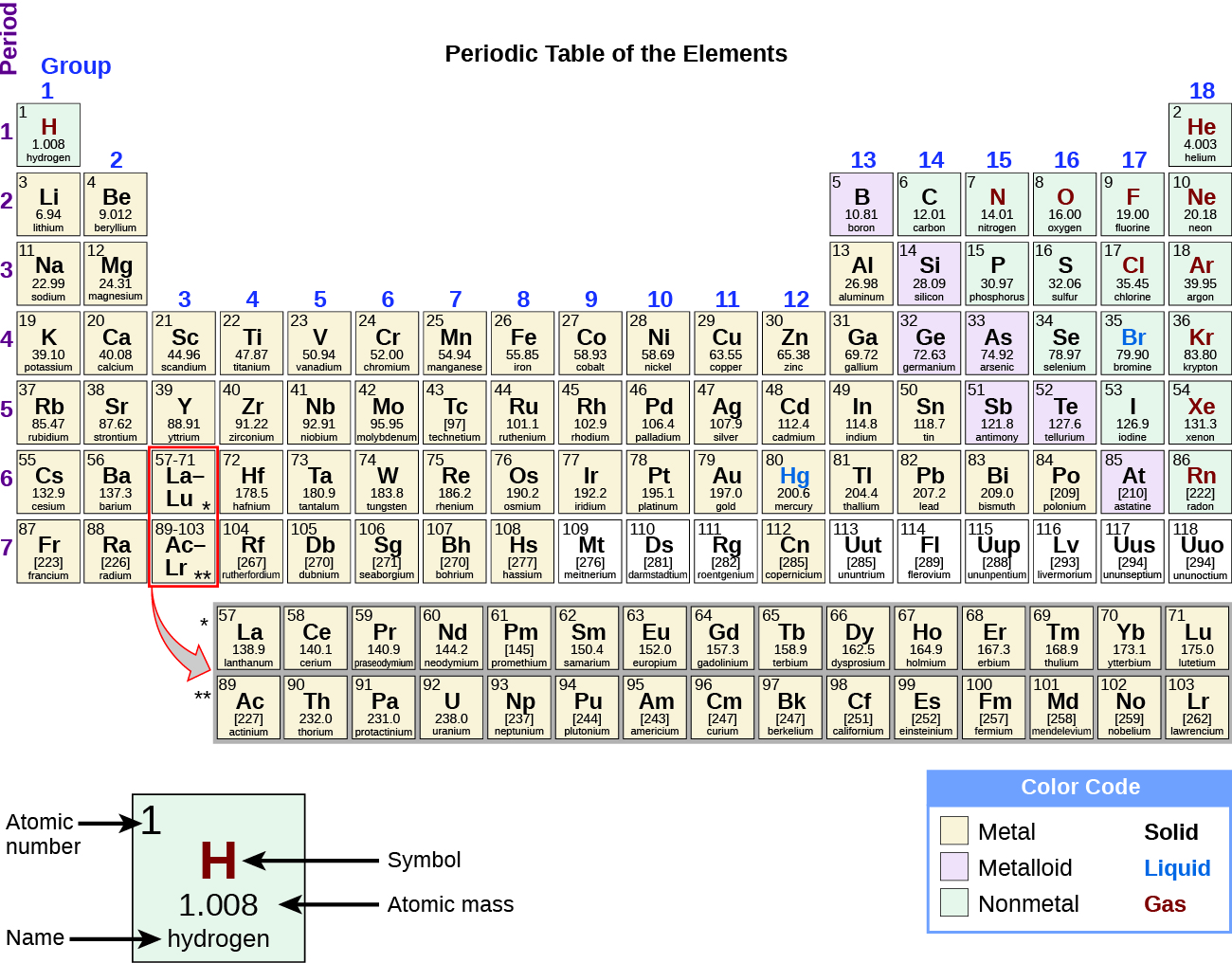
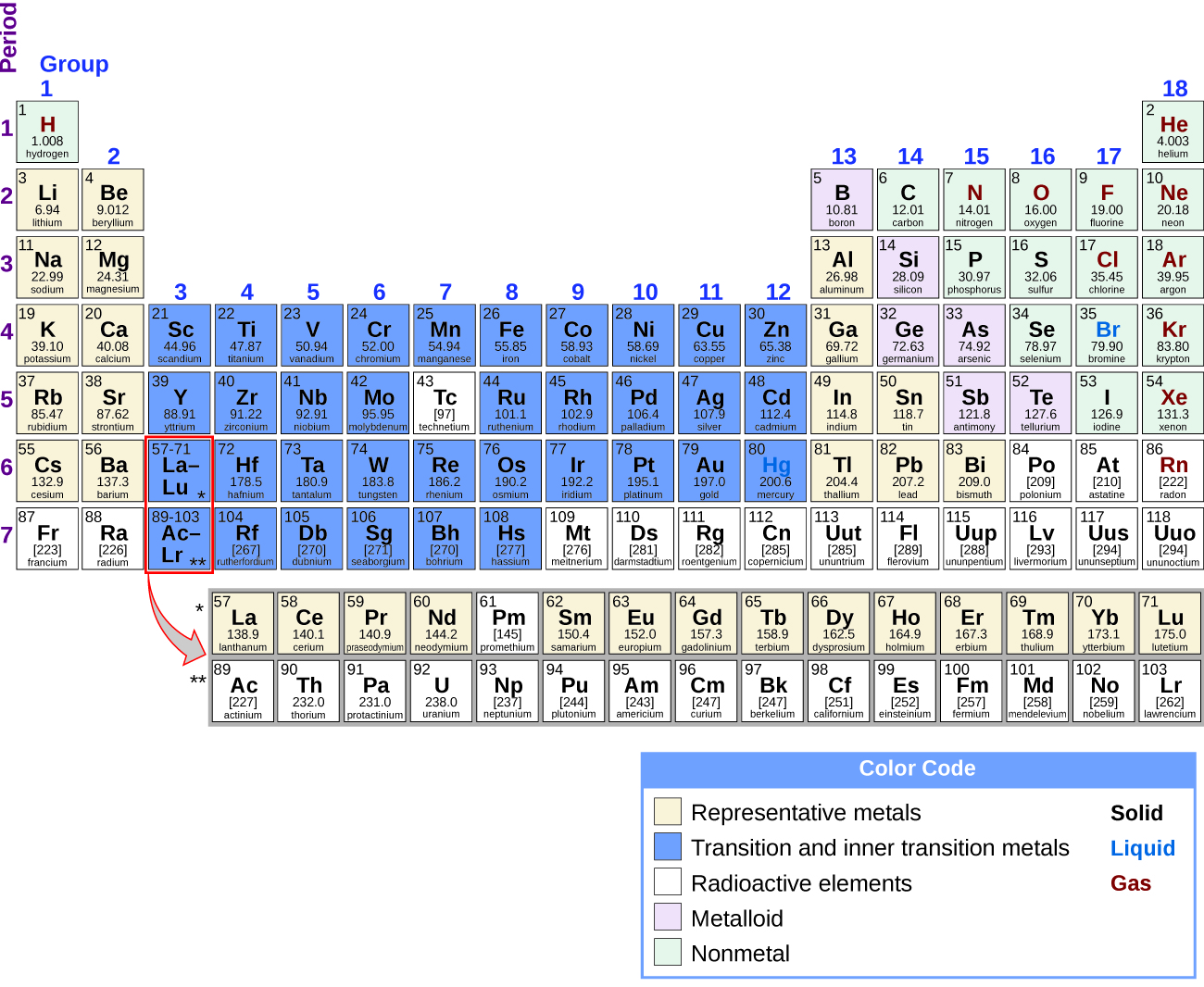
The table below shows the electron configurations of several elements. Most of the compounds formed by representative elements are colourless. Which set of elements should fluorine be grouped with.
205 Electron Affinity 745. In an RC series circuitin fig the rms voltage of source is 200V and its frequency is 50 Hz if. E located in the innermost occupied shell.
Explanation of these properties is organized according to the concept of periodicity with each subsequent section corresponding to one of the eight groups of representative elements. The reaction of these elements with water is very exothermic and can even produce fire or an explosion. Elements of group 121314151617 are reperesentative elements.
The elements get the nearest inert gas configuration by losing or gaining or sharing of electrons. Representative elements are in the group1 group 2 and in groups 13 to 18. Representative elements ____ 9.
The number of valence electrons an atom has determines its location in the period. Their outer shells are not completely filled with electrons. Which statement correctly describes the atoms of the two elements1 point They have the same number of electrons.
They display a wide range of physical and chemical properties. Mn Fe Co Ni Cu. Set A Set B Set C and Set D.
They have valence electrons in the same energy level. A Li b Na c Rb d F e I 4. Xe Rn He Ne Kr a.
Select the term best describing the series of elements. S-lock and P-block elements come under the category of representative elements. Most of them are solids but a few are gases at room temperature and one bromine is a liquid.
Periodic trends provide chemists with a quick and easy tool to quickly predict the properties of elements. Valence electrons relate to the position of elements within the groups and periods of the periodic table and also their position within blocks. A d-transition metals b representative elements c metalloids d alkaline earth metals e halogens 3.
Transition elements are in the groups 3 to 12. The Best Home Tutor. 203a Isoelectronic and Radii 415.
The Representative Elements Group 4A Central element to life Nonmetallic properties Forms Covalent bonds with nonmetals and ionic bonds with metals Small radius allows for the wide occurrence of CC and CO bonds in compounds Central element to electronic technology and artificial intelligences. Arrange the following elements in order of increasing. The element with the electron configuration of Ne3s2 3p4 has six valence electrons so within its period of 3 it must be a group 6.
203 Effective Nuclear Charge and atomic and ionic radii 2322. Which element has the largest atomic radius. A group in the periodic table which includes metals metalloids and nonmetals.
Representative elements include s block and p block elements. 204a Wavelength of light emitted from first excited state 707. Larger atomic size than C.
Two representative elements are in the same period of the periodic table. Choose the term that best describes all members of this series of elements. The representative elements in group one are called the alkali metals and they each possess only one valence electron in their outermost electron shell.
C located closest to the nucleus. The Representative Elements are those elements within the first two families Groups I and II on the far left and the last six families or groups on the right of the Periodic Table. They are chemically active.
Which of the following pairs of elements and valence electrons is incorrect. The elements of s and p blocks except d group elements are called as representative elements.

How The Periodic Table Groups The Elements Live Science
No comments for "Which Best Describes the Properties of Representative Elements"
Post a Comment